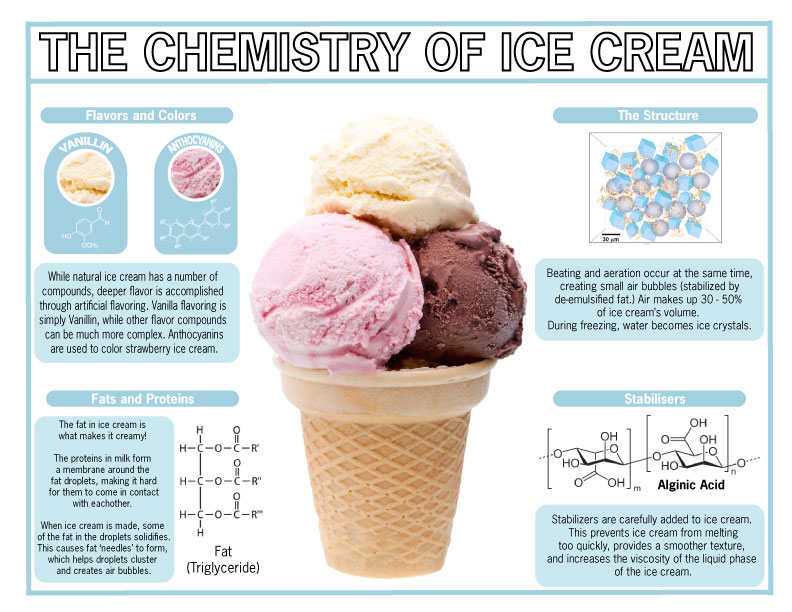
The Chemistry of Ice Cream (yum!)
Email us to request a PDF of this Infographic!
What’s better on a hot day than ice cream? We grew up eating different varieties, textures and flavors… but what is the science behind our favorite summer treat?
Fats, Proteins and Emulsifiers (Oh my!)
Ice cream is simple – its composition consists of water, sweeteners, flavorings (natural and artificial) emulsifiers, stabilizers, milk fat and milk solids.
The richness we taste when we indulge in ice cream comes from the fat content – provided by the milk or cream. When it comes down to physical chemistry, Ice cream has a colloidal structure - this means it’s comprised of small air bubbles and ice crystals dispersed through water and a network of fat globules.
Different emulsifiers are used to make up the surface proteins and help with forming the network of fat globules. When the cream is whipped, the air that’s introduced helps the fat to coalesce and stabilize the air bubbles. After the whipping process, the cream is cooled at a lower temperature than the average freezing point to ensure ice crystals form.
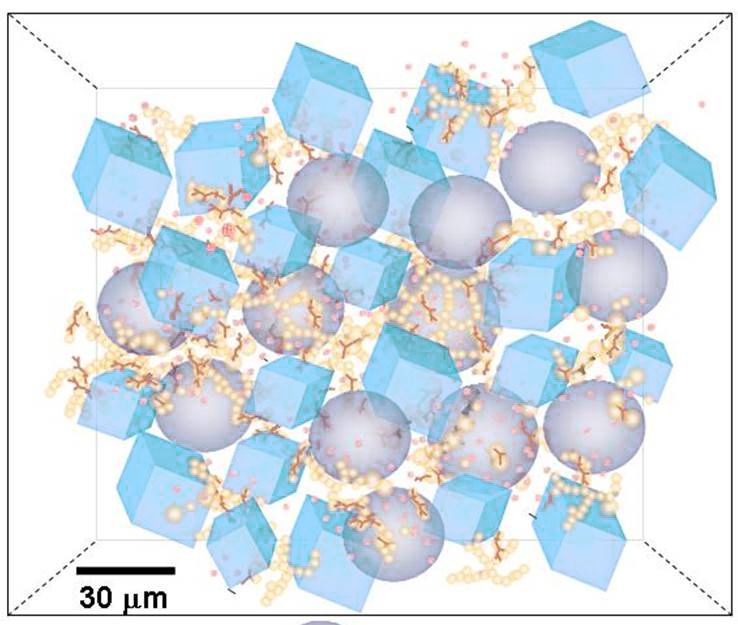
Structure of Ice Cream:
The ice crystals that form when ice cream freezes are integral to the quality of the end product. Soft serve, or smooth ice cream, requires very small ice crystals. To guarantee small crystals, ice cream needs to be frozen quickly. However, ice cream is still made up of 55 – 64% water and when served at an average temperature of -16 degrees Celsius only about 72% of the water is frozen. This allows the ice cream to be “scoopable”.
The Flavors and Colors of Ice Cream:
What makes ice cream taste so good? The flavor chemistry of this creamy treat consists of a complex mix of fatty and watery components – leaving a lot of room for enhancement. Many ice cream manufacturers have to use different artificial flavors (sometimes a mix of three or four) to achieve their signature flavor. A frequently used artificial flavor is Vanillin for Vanilla ice cream. Furthermore, the color of ice cream is important for flavor profiling. Anthocyanins, color pigments found in plants, is what gives strawberry ice cream it’s iconic pink color.
The process of making ice cream is always evolving as food chemists come up with different ways to whip, flavor and freeze the concoction. The next time you sit down to enjoy this cool treat, think about the years of trial and error that have lead up to producing your favorite flavor. What is your favorite ice cream?
Sources:
Halford, B. “Ice Cream: The Finer Points of Physical Chemistry and Flavor Release Make this Favorite Treat so Sweet.” Chemical & Engineering News, Nov 28, 2004: http://pubs.acs.org/cen/whatstuff/stuff/8245icecr... [accessed July 2018].
Recent Posts
-
Disinfecting Surfaces in the Era of Covid and EPA Registered Commercial Disinfectants and Viricides
The disinfection of surfaces at home, in public spaces, and in hospitals and clinics needs to be a …15th Jan 2023 -
Working with Inorganic Acids in the Laboratory: A Practical Guide
Working with Inorganic Acids in the Laboratory Acids are of great importance in the laboratory and a …5th Jan 2023 -
The Top 12 Drinking Water Contaminants
1.Lead- from older plumbing systems pre-1986, when lead pipes, solder, and components were banned. …14th Dec 2022