Chemistry Week

Celebrating the Chemistry of Color
Dyes
Dye is a natural or synthetic substance used to color foods, toys, fabrics, cosmetics, etc. Some are 'food safe' and others are not, but dyes are blends of chemical compounds used in a huge variety of industries:
- Textile manufacturers
- Paint manufacturers & suppliers
- Colorant users, suppliers & manufacturers
- Manufacturers and suppliers of Plastics
- Testing labs, Libraries, academic institutions
- Producers of Printing ink along with suppliers
What makes Dyes colored? Chemistry!
Basically ionising & aromatic compounds, dyes have Chromophores present in them with their structures having Aryl rings that have de-localized electron systems. These structures are responsible for absorption of electromagnetic radiation that features varying wavelengths (as per the energy of electron clouds). These Chromophores make dyes highly effective in their ability to absorb radiation. Further, Chromophores also act by making necessary energy changes in de-localized electron cloud of dye that invariably results in compound absorbing radiation within visible range of colors (not outside it).
Synthetic Food Colors
A color additive is defined as any dye, pigment, or other substance that can impart color to a food, drug, or cosmetic or to the human body. They are classified as straight colors (water soluble), lakes (fat soluble), and mixtures. Also known by name of artificial food colors, these synthetic food colors are processed and manufactured chemically. The FDA regulates color additives used in the United States to make sure chemicals are applied safely, and each one is only approved for certain purposes.
- The name of a lake is formed from the name of the color additive combined with the name of the basic radical and the word "Lake". For example, the name of the lake prepared by extending the aluminum salt of FD&C Blue No. 1 upon alumina would be FD&C Blue No. 1 - Aluminum Lake.
- If a lake is prepared by extending an FD&C color additive on a substratum other than alumina, the symbol "FD&C" will be replaced by "D&C". For example, the name of the lake prepared by extending the aluminum salt of FD&C Blue No. 1 upon a substratum other than alumina would be D&C Blue No. 1- Aluminum Lake.
- For a list of all FDA allowed food colors, visit http://www.fda.gov/ForIndustry/ColorAdditives/ColorAdditiveInventories/ucm106626.htm#list1
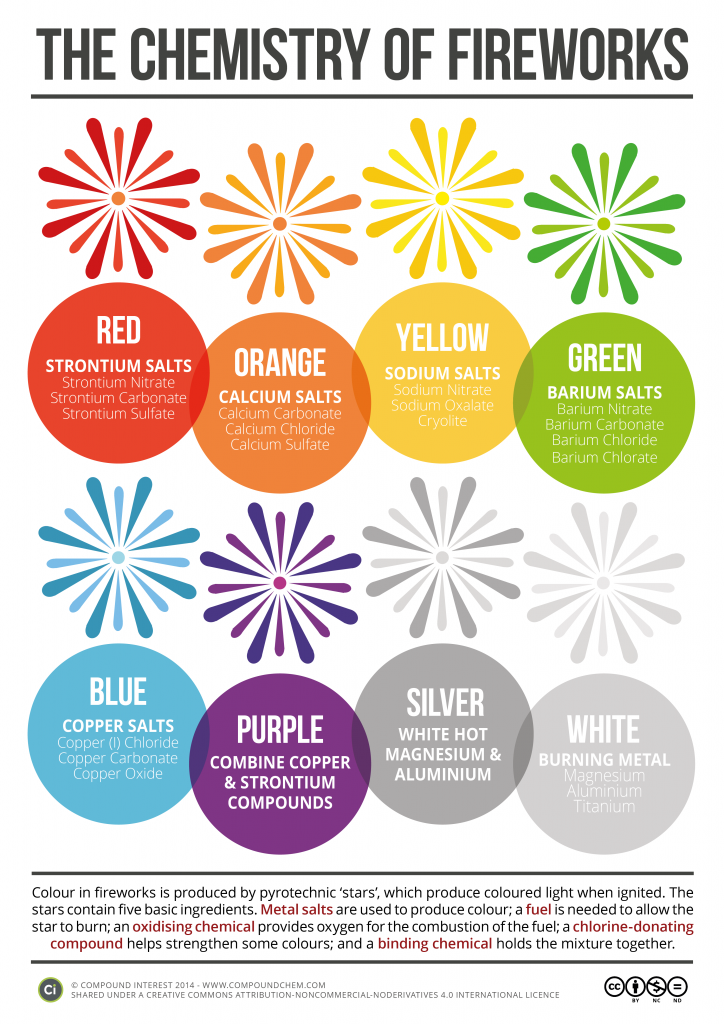
Fireworks
The colors in fireworks stem from a wide variety of metal compounds – particularly metal salts. ‘Salt’ as a word conjures up images of the normal table salt you probably use every day; but this is only one type of salt (sodium chloride); in chemistry ‘salt’ refers to any compound that contains metal and non-metal atoms ionically bonded together, such as calcium chloride or sodium nitrate, and in fireworks they emit characteristic colors.
The atoms of each element absorb energy and release it as light of specific colors. The energy absorbed by an atom rearranges its electrons from their lowest-energy state, called the ground state, up to a higher-energy state, called an excited state.  The excess energy of the excited state is emitted as light, as the electrons descend to lower-energy states, and ultimately, the ground state. The amount of energy emitted is characteristic of the element, and the amount of energy determines the color of the light emitted.
The excess energy of the excited state is emitted as light, as the electrons descend to lower-energy states, and ultimately, the ground state. The amount of energy emitted is characteristic of the element, and the amount of energy determines the color of the light emitted.
For example, when sodium nitrate is heated, the electrons of the sodium atoms absorb heat energy and become excited. This high-energy excited state does not last for long, and the excited electrons of the sodium atom quickly release their energy, about 200 kJ/mol, which is the energy of yellow light.
The amount of energy released, which varies from element to element, is characterized by a particular wavelength of light. Higher energies correspond to shorter wavelength light, whose characteristic colors are located in the violet/blue region of the visible spectrum. Lower energies correspond to longer wavelength light, at the orange/red end of the spectrum.
The colors you see exploding in the sky are produced by the elements with the characteristic emissions listed in this table:
| Color | Compound | Wavelength (nm) | |
|
|
red |
strontium salts, lithium salts |
652 |
|
|
orange |
calcium salts |
628 |
|
|
yellow |
sodium salts |
610-621 |
|
|
green |
barium compounds + chlorine producer |
589 |
|
|
blue |
copper compounds + chlorine producer |
505-535 |
|
|
purple |
mixture of strontium (red) and copper (blue) compounds |
420-460 |
|
|
silver | burning aluminum, titanium, or magnesium |
To make fireworks, the metal salts are formed into "stars" (3-4 cm clumps). Stars consist of a blend of oxidizing agent, reducing agent, coloring agent (metal salt), and binders. When ignited, the stars produce both sound and light effects. The appearance of a firework is determined by its stars, which are made by hand. Read more at http://scifun.chem.wisc.edu/chemweek/fireworks/fireworks.htm
Sources:
- http://www.fda.gov/ForIndustry/ColorAdditives/ColorAdditiveInventories/ucm106626.htm
- https://learningcenter.nsta.org/products/symposia_seminars/fall08/FDA/webseminarI.aspx
- http://www.wisegeek.org/what-is-food-coloring-made-of.htm
- http://www.foodcolorworld.com
- http://scifun.chem.wisc.edu/chemweek/fireworks/fireworks.htm
- http://www.compoundchem.com/2013/12/30/the-chemistry-of-fireworks/
Recent Posts
-
Safety First: Choosing the Right PPE for Your Laboratory
Are you aware that over 20,000 workplace injuries occur annually i …1st Oct 2025 -
The Future of Lab Supplies: Trends You Can't Ignore
As laboratories across various industries evolve, the demand for i …29th Sep 2025 -
Ergonomics in the Lab: Choosing Equipment for Comfort
In the fast-paced world of laboratory work, comfort should not be …15th Sep 2025
